Cracking oil is not a funny business
Posted by Heading Out on March 26, 2006 - 1:54pm
For those new to the site this is where, on weekends, I often post a small technical talk, explaining some of the aspects of the fossil fuel business, so as to help understanding of some of the topics on the site. There are now two main topic themes developed, those relating to oil, and those to coal. Since this talk relates to oil, at the end I will post the list of topics that relate. It is a very simple explanation, because of space, and those who wish to ask or expound a bit more are invited to do so through the comments.
For example, of one looks at the blue line in the following graph, this shows a typical light oil composition, and might well yield, on passing through the first distillation column of a refinery, the sort of separation that I showed in the plot last time.
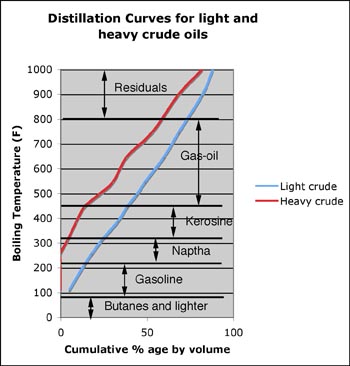
However, as folks are pointed out, we are seeing less of these light oils on the market, and increasingly we must use heavier crudes. To make the point consider the red line in the same graph.
You can see that there is no fraction boiled off until the temperature has passed 250 deg. Which means that, in its untreated state, there is no natural gasoline in the oil. Likely it was too close to the surface and those fractions evaporated away over the millennia (as is the case with the heavy oils in Alberta for eg, though this example is from somewhere else). So how do we change this mix into something more useful?
The answer, not only for this crude, but also for the heavier fractions of the light crude, since in both cases we would like to end up with about 45% gasoline, requires that we crack (or split) the higher carbon molecules into lighter or lower carbon ones. For example say we have a molecule of Cetane (16 carbon & 34 hydrogen atoms or C16H34) if we heat this to a high enough temperature, then we can break it down into some Octane (C8H18), some Hexane (C6H12) and some Ethylene (C2H4). But, obviously, what we would like to do is to control what it is that we break these heavier molecules into, and how much of each we produce.
And so we get into the world of Cracking Oil. After the crude oil has been separated into the different fractions, the gas-oil fraction is then fed to a second heating process, where the fluid is brought back up to a high temperature (perhaps around 735 deg C 1400 deg F) and mixed with a finely powdered catalyst (hence the "cat"). Steam is also added to help with the movement of the mix, and the combined mix is fed up a pipe (called a riser) into a tank and as it flows up and into the tank the gas-oil breaks down into the lower carbon molecules. This happens very quickly, so that by the time the mix is in the tank, the heavy molecules have broken down, and so can be drawn off. However the catalyst has first to be removed, which happens by passing the flow through cyclones that spins the flow and separates the heavy catalyst, which is collected and fed down a pipe back to be reclaimed and re-used. It needs to be processed since, in part, the process is not perfect and carbon will be deposited on the particles of catalyst, blocking its action. (It can be burned off - providing some of the heat for the process). The catalyst can then be re-used. One operation might use the catalyst at a flow rate of some 55 tons/minute.
The hydrocarbons that flow out of the cracking process are then separated into different fractions, depending on the season more gasoline or distillate might be desired, but the process might yield about 8% coke, 55% gasoline, and 12% light gas oil with about 10% of the flow being a sufficiently heavy product that it is sent back to be run through the process again. Light gas oil becomes diesel and furnace fuel oil. The lighter gas products are usually sent to alkylation or reforming where the lighter molecules can be combined to give products that can be blended into gasoline. (I got this wrong in last week's post).
Different crudes have different mixes of hydrocarbons, and will need different sizes of processing sections that convert these different fractions to usable and desirable products. Refineries havee been built to usually handle only a given range of crudes, and thus have to be considerably modified if that mix should change. Sometimes the crude can be even heavier, the sands in Alberta contain an oil which is more conventionally called a bitumen, since it has less volatile material that the heavy oil example I used. That oil must therefore go through an additional step to produce the lighter synthetic oil that can then be distilled and cracked more conventionally. Some of the new processes involved in mining and processing the ore are described in a pdf file by Herron. There are also contaminants such as sulfur which can be found in the oil. If the content is higher than 2.5% then the oil is called "sour"; this sulfur must be removed, and while at one time it was used as fuel in the refinery that has been stopped and now it is recovered. That also requires a treatment circuit that must be installed if the mix to the refinery changes.
There is a pdf from BAH here that explains the impact that some of these changes in supply may have.
This is part of an ongoing weekend series on technical aspects of oilwell (and natural gas) drilling. Previous posts can be found at::
the drill
using mud
the derrick
the casing
pressure control
completing the well
flow to the well
working with carbonates
spacing your well
directional drilling 1
directional drilling 2
types of offshore drilling rigs
coalbed methane
workover rigs
Hydrofracing a well
well logging
seismic surveying
gravimetric surveying
carbon dioxide EOR
refinery distillation
As ever, if this is not clear, or if there is disagreement then please feel free to post, and I will try and respond.



Can you give some estimate on how much energy is lost during the refinery process and what happens to that number when crude is of a more heavy grade.
I guess what I'm asking is: Is there, after cracking/refining, as much gasoline in a light sweet barrel as in a hevy crude barrel?
(Im not dumb, Oil is just not my trade....)
One way of phrasing what Im getting at is:
When the gasoline is in the hummers tank ready to be consumed; Is there already a larger CO2 price tag on the gasoline derived from Heavy than on gasoline from light sweet.
How much of these heavy oils are currently being "cracked" and how much are used as is for lubricants or chemical/plastic production? I took organic chem almost six years ago but are the heavy liquids not more valuable for producing goods than burning?
matt
In order to read the paper, it is necessary to know what coking is in addition to cracking. Do you have anything to add to your comment I just referenced, HO?
Again, thanks for the info.
How much natural gasoline is in light sweet? I assume it must vary from well to well? Comparing a barrel of light to a barrel of heavy crude, what is the approx. percent loss in the barrel of heavy oil? Lastly, do the two barrels contain the same number of BTU's, the same EROEI?
Thanks...
light, medium, heavy
30%,56%,14%
going in 2010 to:-
31%, 58% 12%
They also see WTI crude prices ranging $50 to 30$ in 2010 with US demand ranging from 2% growth to 2% fall due to the adoption of hybrids and ethanol and see overinvestment in refining as a real danger leading to reduced refining margins.
If this is the consensus in the refining industry and what seems to be the consensus here on TOD is true that prices will rise, the percentage of heavy sour crude will rise and Jevons paradox will take the shine off most improvements in oil consumption/mile of vehicles there seems to be a high risk of an even greater US refining bottleneck.
In general, this is a strange problem that I myself do not pretend to understand.
What's the opinion of the experts here? Is it possible to crack oil with sound waves in an economic way?
Is it possible to crack oil with sound waves in an economic way? Beats me.
I'm old enough to have grown up on those popular science for kids type books the US was flooded with as part of the Space Race. Every book on transportation, and there were plenty of them, had its bit on how crude oil is found (all those neat cross-sections of the earth showing oil and gas pockets) and cracking towers. It generally showed nat. gas being burned off too, pesky stuff.
Yes, a bbl of oil != a bbl of gas for your tank. There's everything from natgas to asphaltum in there, and the heavy sour has more of the nasties and less nice light stuff in it.
This news article provides some useful context. It suggests that the world's most profitable refinery achieves that through a very flexible approach to cracking, and handling differing crude varieties.
Worth a read if you've got this far in the thread.
Cheers,
--J
Anyone out there can confirm light sweet has passed its' peak?
Anyway, this post goes straight into my bookmarks for further evaluation of all links provided. thanks HO.
A friend of mine who used to be in the drilling business here in Texas claims that there have been oil wells yielding lighter fractions that could be burned directly as gasoline. These were known as white gas wells.
One aspect of the refining process that has mystified me is the reason that all refineries must be shut down for regular maintenance, and which causes major delays and problems. The refineries sometimes blow up when started up again. Why is this maintenance needed? Does carbon perhaps build up in the catalyst and need to be burned away, or does something else build up and need to be removed?
Part of the essence of refining, as I understand it, is that the standard way to improve both heavy crude and sour crude is to add hydrogen, best made from natural gas. This reduces the sulfur, which precipitates out, and also makes it possible to crack the heavier hydrocarbon chains into lighter, shorter ones needed for gasoline. But it takes the hydrogen to do it. You would probably rather use natural gas than the more locally available heavy crude to make the hydrogen.
As a general trend, there seems to be a tendency for the refining operations to move closer to the oil supply. The Saudis are going to invest heavily in refining capacity over the next decade, as I understand it.
The vast concentration of refining and petrochemical facilities around the Texas Gulf Coast seem very vulnerable, due both to stronger hurricanes and due to the refining and to processing gradually moving downstream and nearer to the sources of hydrocarbon production. -- Roger
Knowing about a ship's steam propulsion system, it's easy to extrapolate that a refinery is going to be a high-maintenance device. Many components are shared, though not configured the same of course. Don't forget the sour crude is acidic. Heat up an acidic substance, and it gets more acidic. Acidic carbon deposits are a greenie's nightmare! Yecchh!
Hope this helps...
Add to this that refineries in the U.S. are long past their original design lifetimes. After twenty or thirty years stuff like copper tubing used for instrumentation lines becomes brittle and develops leaks. Pipe vibration will eventually cause expansion joints to fail if you ignore them.
Most of the refineries are also located near large bodies of salt water. You've got miles of carbon steel piping, dozens of steel equipment skids, and more steel cabinets and enclosures than you want to know about. All of which must be continuously painted or they rust out within years.