Separating Different Components from a Crude Oil
Posted by Heading Out on January 3, 2010 - 11:04am
In this series of tech talks, I have been writing about the progress of crude oil as it passes from the reservoir up through the production casing, and out to the GOSP, where water, oil, natural gas, sand and sulfur products can be separated. The example video that I referred to last week dealt with treating Canadian Oil Sands, and the oil that is coming from them.
What I want to talk about today is the differences that exist in what to some folk is just "crude oil," with the assumption that it is all the same. In writing about coal, it is fairly simple to show that the different stages of coal as it changes from peat to anthracite. This means that you get different amounts of energy from it, and it can be extracted with differing amounts of energy. The fact that there is a fair bit of difference in crude oils is not always as easily understood. This then will be a relatively simplistic look at the different potential hydrocarbons that might make up a crude oil, and how we can get them apart.
Crude oil is made up of a mixture of hydro-carbons, which are the different ways in which carbon and hydrogen can combine, starting with such simple compounds as methane (CH4) and progressing to more complex ones with greater numbers of carbon atoms.
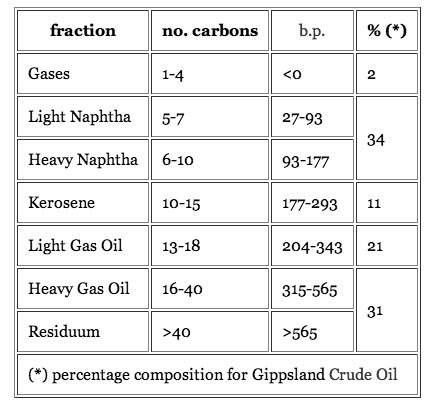
Oils from different places have different combinations of the major constituents, for example, this is from Kuwait.
Because they are fluids mixed together, it is not very easy to separate out the different valuable parts (known as fractions) by a mechanical means. However, if you heat up the crude oil blend, then all the constituents will vaporize.
But the different fractions of the oil will boil at different temperatures (or boiling points ( b.p.)), at which point they turn into gas. And so the first part of the treatment that the oil gets, when it reaches a refinery is that it is heated, so that it will all turn into such a gas, and then it is cooled in stages, so that the different fractions will condense back out. The total process is known as crude oil distillation and the UK Schools site has a simple sectional picture of what such a distillation column might look like.
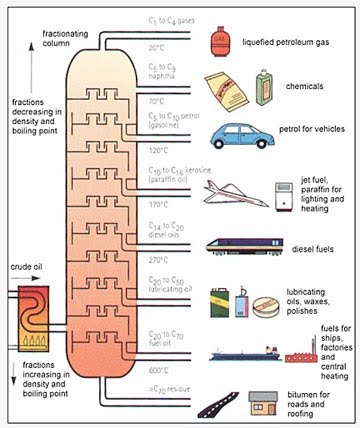
As the combined vapors from the heated crude enter at the bottom of the tall tower (called a column), they pass up through different trays that are placed at set heights up the column. When the gas reaches a tray it passes up through it into a bubble cap, this is a cover over the hole that pushes the gas down so that it has to bubble up through the liquid that has already condensed onto that tray.

The liquids in each tray, as the vapor rises higher in the column, are kept at lower temperatures, so that the heavier oils, that condense at a higher temperature, will condense lower down the column. As the lighter vapor rises through successive trays, the temperature of the liquids drops, and lighter fractions of the oil also begin to condense out, until the very lightest are collected at the top, still as gas, and are fed on to a cooler. The liquids then drain, either back down to a lower tray, or through a side-draw pipe that taps the fluid from the trays and takes it away for either further division or for storage and sale. A typical initial distillation might yield:
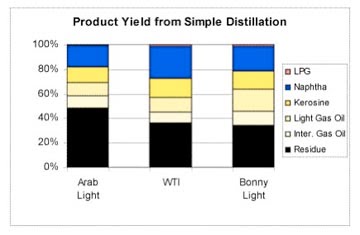
Each year the EIA publishes its world distillation capacity which is the necessary part of getting from crude to useful product.
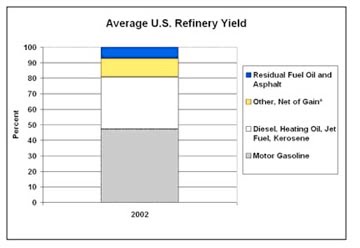
I will continue this further in a later post, talking about the further stages in refining, and cracking of compounds to break them into lighter fractions, so that the next product from a refinery might at the end, look something like this (courtesy of the EIA).
I hope that each of you has a Prosperous and Successful life in the year ahead.



Many thanks for this.
From earlier posts I've learned that the sulphur content ends up as a major contribution to world agricultural sulphur supplies.
Is there any particular reason that crude oil contains nickel and vanadium? Does it vary from source to source? Are there other trace metals that might be useful?
...and a happy and resourceful New Year to all TODers....
BobE
The metal and other elemental contents of the different crudes vary considerably with the original source, and the various other stages it has passed through to get to where it is today. The volumes that are generated have to be considered in terms of the overall volumes produced, relative to the costs of generating the pure product, to determine what will be marketable. And that too varies from site to site.
The metals content of crude oil is too low to warrant recovering them. After the crude is boiled (fractionated) it is fed through reactors full of catalyst. The first catalyst it comes in contact with absorbs the metals because the next bunch of catalyst is designed to break long hydrocarbon chains apart and metals will destroy the active sites on the catalyst. You can think of the catalyst as little pills made up of some sort of support structure sort of like sand in some cases, made of aluminum in others. The surface of the catalyst has some sort of metal (platinum, copper, nickel, or molybdenum for example) which help initiate the desired reactions. The catalyst is extremely expensive and the amount of metal in the catalyst is greater than the amount it can remove in the feed. It's just not worth it to try to recover the metals off the catalyst - there is no easy way to do it. Sulfur is different because there is a significant amount of it in the crude oil ~2-3% and unlike the metal contaminants, it can be recovered and in fact has to be recovered because in the vapor form (as H2S) it causes acid rain. Elemental sulfur is the only product refineries sell at a loss because they have to extract it but have no where to put it so have to get rid of it to anyone who will buy it.
Thanks for this. It's just that in the past I was wondering where all the rare earths were and was surprised to find that coal ash contains a reasonable proportion of gallium. I thought maybe there might be some useful elements lurking in oil.
H.O., I find these tech talks very helpful.
As I understand it, smaller hydrocarbons flow more easily than complex hydrocarbons - especially the asphaltenes - and water flows more easily than most (the vast majority) of the liquid hydrocarbons. I also understand that the heavier, more complex hydrocarbons are also more likely to carry more impurities (especially sulfur).
So, am I correct in deducing that as the years go by, the crude stream produced from a single reservoir becomes heavier and more sour? If so, is my hypothesis correct? If not, why not?
Thanks in advance!
Because of the way that the oil is held in the rock formations underground there is not a whole lot of mixing that goes on in the reservoir itself, and it is that mixing, with subsequent stabilization, that would give you a gravity separation in the reservoir. As a general rule that does not happen, and so you would get a similar hydrocarbon mix coming out near the end of the well life as at the beginning, with a couple of significant caveats.
The first of these is that the driving pressure in the reservoir will drop as oil is produced, and this can cause the gas that is dissolved in the oil to come out and form a gas cap over the oil. The other is that with age, and particularly if water is being used to sustain pressure and drive the oil then the water cut of the product will rise. (Though it can be controlled in part by blocking off lower sections of the well as they become further below the oil:water line).
Just to say how much I appreciate these posts. A glass of wine, a reading of Heading Out, and I go to bed on Sunday evening a relaxed and more knowledgeable person.
Another way to crack crude is run it through a large furnace. At the outlet are quench rings which spray oil into the stream to almost instantly drop the temperature from say about 1000 deg F to 400 deg F. The naphthas drop out after the quench ring.
I always thought this subject was interesting.
As I understand it, US oil that was produced early on tended to be quite light (and sweet). So we built our cars to use gasoline, since that was the predominant product produced. It had some other good qualities as well.
Heavier oils, like that from Kern River, produce mostly products at the heavy end of the scale. Without upgrading (which I think Dave will get to next week), one ends up with a lot of asphalt, and not much light products. So the oil is upgraded in more expensive to operate refineries that do "cracking".
The world is now getting to the oil that was bypassed the first time around, as being too polluted with vanadium and nickel and sulfur. More advanced separating and refining facilities are being built, to handle the particular issues of some of these oils.
The highest energy products per unit of volume are the heavier products. This is why diesel has more energy per gallon than gasoline.
I've always wondered about the split that seems to have happened between the US and Europe with Europe favoring diesels over gasoline. With the US importing a lot of gasoline not only from Europe but also Asia.
Why did it happen how did it happen etc.
As world oil production continues to decline will we more likely see economic or bureaucratic unavailable of gasoline, diesel fuel or electricity . Would it be wise to buy one of the new low pollution VW Jetta diesels a Prius or wait for some new exotic. My current sedan is getting old and decrepit.
There are a lot of politics and other outside controls tied into trying to give an honest answer, but let me just say that over the past couple of years we have traded in the two cars that we own. (They were each around ten years old, but we didn't qualify for the Clunkers program). In both cases we bought hybrids (Toyota Camry and Ford Focus) with the selection based in part on reviews we read. We have been delighted with both purchases. But then we live in a small town where if you want to get a diesel serviced (and two of my friends had them) you have to drive over a hundred miles to a dealer.
I have a 5-yr old VW diesel and love it. However, in an emergency diesel may be reserved for essential transport. If your fuel system is not compatible with 100% biodiesel or a "greasel" conversion you may be out of luck.
Believe it or not, in the early days there was a time when gasoline was considered a waste product, a nuisance to get rid of, just like sulfur is today.
Thanks for these Tech Talks, Heading Out. I find them very informative. I've been wondering for a while exactly what constitutes Naphtha and your charts shows both light and heavy Naphtha from 5-10 carbons in length. Am I correct that the light Naphtha is basically what we call "White Gas" or Coleman Fuel? Also, is the Heavy Naphtha fraction what is turned into gasoline? I've read in the past about natural gasoline coming from oil wells, and apparently in the "old days" it was sometimes used straight in cars.
Thanks again,
Larry
I will defer on this to someone who might be able to give a more accurate answer than I would.
This is thorny subject. Ina refinery there are many naphthas ( virgin, FCC, coker,hydrocracked). Different types of crude oil will yield different quality naphthas but in the main virgin strainght run naphthas will contain a mixture of paraffins, cycloparaffins and aromatics. Most petrochemical grade naphthas normally have a minimum paraffin content of 70% by weight with a typical range of 65-75 % weight. It very much depends on the crude type. Gas condensate and natural gasoline, which is produced from natural gas and associated gas would normally have an even higher paraffin content ( ie Saudi Aramco A180 grade or ADNOC paraffinic naphtha).
For illumination and cooking purposes a light paraffinic aromatic depleted naphtha would be prefered boiling in the range of around 95 to 110 deg C.Exxon makes such grades as Isopar C, heptane and at a stretch DSP 100/120, details of which can be foumd on the Exxon website ( other manufacturers include Shell, Conoco Phillips, Total and DHC). Exxon also produce some refined mixed napthas called Varsol grades, and aromatic (95% +) naphthas called Solvesso grades. The solvesso grades are produced from Reformate. The other manufactures above do likewise but to a lesser degree. Natural or wild gasoline is the C5-C7 fraction produced from crude oil stabilisation which is the heavy fraction of NGL's (see reference to associated gas)
There are a few rules of thumb. The heavy naphtha fractions tend to be more aromatic and the lighter naphtha less aromatics (aromatics start at C6). For a given boiling range the flash point and desnsity will give a good clue on the aromatic content. The higher the density and flash point, the higher the aromatic content. This is due to the hydrogen content and the intermolecular forces, the latter greater for aromatic species. Look up UOP k Factor
In a refinery the virgin naphtha is normally split into light and heavy at around 90 -95 deg C . The heavy naphtha is used as reformer feed and at this cut point most of the benzene precursors have been removed. The light naphtha is used for mainly petrochemical feedstock in Europe but in the US more is feed to isomerisation units for the production of gasoline isomerate, rich in isoparaffins.
In the US per barrel yield of gasoline is abot 40% of the crude barrel. In Europe it is much less at <25% due to the increasing dominance of the diesel engine in automotive applications.This is unlikley to change even with the growing availability of hybrids.
Please note I do not work for Exxon and never would. I know their products well and have used them to illustrate the differences.
There are many different options the refiner has when it comes to how he wants to split up the crude stock. It is always dependent on the price for which he can sell the products. All refineries have some leeway in the amount of each product they can produce within limits. Some refineries are constructed to have very good flexibility and some are not very flexible.
To get to your question, the word naphtha, when when used by a refiner, generally refers to the mixture of hydrocarbons which boils in the gasoline range. Everyone has different definitions for what light naphtha is, what heavy naphtha is, etc. Some refineries don't make a distinction, some do. The thing that everyone agrees on are the specific qualities of the final product. Naphtha is not specific, 87 octane gasoline is. Gasoline has many requirements placed on it for environmental and performance reasons. Some hydrocarbon components have a high octane which allow for higher compression before they ignite (good for performance engines) but are carcinogenic - benzene is a good example. Some really help the gasoline flow properly in the cold but evaporate quickly in the summer so there are regulations on how much of it can be blended into the gasoline in order to cut down on smog and reduce explosions at gas stations. Heavy and light naphtha both make up parts of the gasoline blend. I am not particularly familiar with what white gas is exactly, it is such a small portion of the products stream it never gets any love. "Natural gasoline" is now generally called straight run naphtha meaning the crude oil has been boiled and whatever boiled in the naphtha range was collected and put directly into cars. We don't do this anymore because there are laws saying it has to be treated and further separated to remove carcinogens and toxins and to ensure it has the appropriate properties. This is obviously much much better for the environment but the restrictions do make gasoline more expensive. The same can be said for diesel (ultra low sulfur diesel costs alot more to make than the old high sulfur diesel).
In general we should expect heavier feed stocks in the future meaning that either less of the products will be in the naphtha range - a higher percentage will be diesel, fuel oil, or asphaltenes - or more complex (read expensive) refinery units will need to be built which can break the long carbon chains into smaller ones which will boil in the naphtha range.
Yes, Coleman Fuel/White Gas does come from naptha.
According to the Wikipedia article, Coleman Fuel b.p. is 47C. I am guessing that this is a specific sub-fraction of the total fraction classified as "Naptha".
"White Gas", sold as Coleman Fuel, is plain old unleaded gasoline with no additives. It is "white" because no road tax dye is added.
Love these articles! Love the comments, too!
Thanks!
Bob
Coleman fuel also probably does not meet octane or other requirement for motor gasoline. However, we used to get rid of old Coleman fuel by dumping it into the gas tank of an old car or truck. As long as you mix it with enough good gas, a bit of substandard gasoline in the tank makes little or no difference unless you're driving a Turbo Porsche.
On the other hand, don't use motor gasoline in your Coleman stove unless you enjoy being poisoned. A lot of the additives in motor gasoline are toxic. People often assume that refineries remove the toxic substances from crude oil when they produce gasoline. No, they add more toxic substances than they remove.
I second that warning. Years ago I was out of Coleman fuel so I siphoned a little gas from my pickup. The regular unleaded burnt fine in the camp stove, but the one use made us nauseous for a day or two, and the food picked up some of the taste too, never again.
How the world's refineries are coping with what the market has to offer and what we can expect in the future relative to the ever changing grades of crude oil is a question I would think could explain much relative to where the real "fuel" oil supply is going to end up?
Thanks for another informative post for us laypersons.
This is great stuff! Thanks.
Would you do a later post detailing additives, things that are added to the oils entering the stream, and how they increase the volume and are added to the total liquids figures we see all the time. Thanks again.
I once climbed in a fractionating column at the Coryton oil refinery to clean it out. It absolutly doesnt look like what you would expect from looking at text book examples :)
Thanks for the crisp refresher on Crude and its yield composition. Please continue to provide similar briefs on oil. For example I would be happy to read on Euro fuel classifications